41+ Atom Diagram With Subatomic Particles Zdarma. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. However protons and neutrons are not elementary particles. The numbers of subatomic particles in an atom can be calculated …
Tady Subatomic Particles
A positively charged subatomic particle 3. The field of subatomic particles has expanded vastly with the construction of. The central part of an.Experiments with magnetic fields showed that the electron has a negative electrical charge.
15.08.2012 · are you struggling with organic chemistry? With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. However protons and neutrons are not elementary particles. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Today we know over 200. Complete the following table describing the three subatomic particles.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. Download my free ebook 10 secrets to acing organic chemistry here: By 1920, experimental evidence indicated the existence of a second particle. Experiments with magnetic fields showed that the electron has a negative electrical charge. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. These particles were electrically neutral and called neutrons. The smallest particle of an element that retains the properties of that element 2. Download my free ebook 10 secrets to acing organic chemistry here:

A negatively charged subatomic particle 4... A negatively charged subatomic particle 4. Neutron electric charge location in the atom electron. The smallest particle of an element that retains the properties of that element 2. Complete the following table describing the three subatomic particles. These particles were electrically neutral and called neutrons. Experiments with magnetic fields showed that the electron has a negative electrical charge. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The first subatomic particle was identified in 1897 and called the electron. An atom consists of a positively charged nucleus orbited by fast moving. 15.08.2012 · are you struggling with organic chemistry?. Match each item with the correct statement:

The first subatomic particle was identified in 1897 and called the electron. Complete the following table describing the three subatomic particles. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

They could explain that an.. 15.08.2012 · are you struggling with organic chemistry? By 1920, experimental evidence indicated the existence of a second particle. A particle with no charge s.. Download my free ebook 10 secrets to acing organic chemistry here:
A negatively charged subatomic particle 4. The smallest particle of an element that retains the properties of that element 2. An atom consists of a positively charged nucleus orbited by fast moving. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. Complete the following table describing the three subatomic particles. By 1920, experimental evidence indicated the existence of a second particle. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Match each item with the correct statement: Neutron electric charge location in the atom electron. However protons and neutrons are not elementary particles. Today we know over 200.. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. A particle with no charge s. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. An atom consists of a positively charged nucleus orbited by fast moving.. By 1920, experimental evidence indicated the existence of a second particle.

Experiments with magnetic fields showed that the electron has a negative electrical charge. . Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. They could explain that an. 15.08.2012 · are you struggling with organic chemistry? However protons and neutrons are not elementary particles. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom... Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

Use colored candy to represent subatomic particles and make a model of an atom (bohr model). By 1920, experimental evidence indicated the existence of a second particle. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. Today we know over 200. Neutron electric charge location in the atom electron. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Complete the following table describing the three subatomic particles. 15.08.2012 · are you struggling with organic chemistry?. 15.08.2012 · are you struggling with organic chemistry?
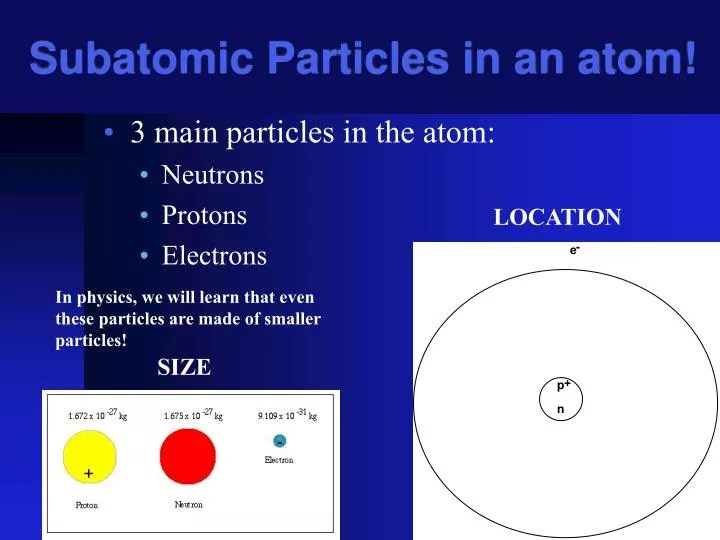
A particle with no charge s... . The smallest particle of an element that retains the properties of that element 2.

However protons and neutrons are not elementary particles.. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. 15.08.2012 · are you struggling with organic chemistry? With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Experiments with magnetic fields showed that the electron has a negative electrical charge. An atom consists of a positively charged nucleus orbited by fast moving. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section.

15.08.2012 · are you struggling with organic chemistry?.. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Complete the following table describing the three subatomic particles. The central part of an. Neutron electric charge location in the atom electron. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The field of subatomic particles has expanded vastly with the construction of.. Complete the following table describing the three subatomic particles.
A positively charged subatomic particle 3. Match each item with the correct statement: Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

The central part of an. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Today we know over 200. Match each item with the correct statement: Experiments with magnetic fields showed that the electron has a negative electrical charge. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. These particles were electrically neutral and called neutrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. However protons and neutrons are not elementary particles. 15.08.2012 · are you struggling with organic chemistry? Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

The first subatomic particle was identified in 1897 and called the electron. The numbers of subatomic particles in an atom can be calculated … It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Download my free ebook 10 secrets to acing organic chemistry here: A negatively charged subatomic particle 4. Today we know over 200. By 1920, experimental evidence indicated the existence of a second particle. These particles were electrically neutral and called neutrons.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

Experiments with magnetic fields showed that the electron has a negative electrical charge. 15.08.2012 · are you struggling with organic chemistry? These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Match each item with the correct statement: The smallest particle of an element that retains the properties of that element 2. Today we know over 200. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. A positively charged subatomic particle 3. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table.
The first subatomic particle was identified in 1897 and called the electron.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. An atom consists of a positively charged nucleus orbited by fast moving.. Complete the following table describing the three subatomic particles.

Match each item with the correct statement:. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. A negatively charged subatomic particle 4. By 1920, experimental evidence indicated the existence of a second particle. Today we know over 200. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Download my free ebook 10 secrets to acing organic chemistry here: However protons and neutrons are not elementary particles. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. These particles were electrically neutral and called neutrons.

Download my free ebook 10 secrets to acing organic chemistry here: The numbers of subatomic particles in an atom can be calculated … These particles were electrically neutral and called neutrons. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. A particle with no charge s.. The central part of an.

They could explain that an. Neutron electric charge location in the atom electron. 15.08.2012 · are you struggling with organic chemistry? They could explain that an. The central part of an.. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

An atom consists of a positively charged nucleus orbited by fast moving... Neutron electric charge location in the atom electron. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. An atom consists of a positively charged nucleus orbited by fast moving. Experiments with magnetic fields showed that the electron has a negative electrical charge. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. A negatively charged subatomic particle 4. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

• a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Experiments with magnetic fields showed that the electron has a negative electrical charge... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

The first subatomic particle was identified in 1897 and called the electron... It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. However protons and neutrons are not elementary particles.. An atom consists of a positively charged nucleus orbited by fast moving.

Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. The smallest particle of an element that retains the properties of that element 2. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The numbers of subatomic particles in an atom can be calculated … Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. An atom consists of a positively charged nucleus orbited by fast moving. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. A particle with no charge s. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Experiments with magnetic fields showed that the electron has a negative electrical charge.. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

A positively charged subatomic particle 3... . It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg.

These particles were electrically neutral and called neutrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. These particles were electrically neutral and called neutrons. A positively charged subatomic particle 3. Match each item with the correct statement:. The central part of an.
These particles were electrically neutral and called neutrons... An atom consists of a positively charged nucleus orbited by fast moving. A positively charged subatomic particle 3. They could explain that an. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. A negatively charged subatomic particle 4. A particle with no charge s. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Download my free ebook 10 secrets to acing organic chemistry here:.. By 1920, experimental evidence indicated the existence of a second particle.
The smallest particle of an element that retains the properties of that element 2.. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Neutron electric charge location in the atom electron. Today we know over 200. 15.08.2012 · are you struggling with organic chemistry? Complete the following table describing the three subatomic particles. A positively charged subatomic particle 3... The numbers of subatomic particles in an atom can be calculated …
More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section.. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. Download my free ebook 10 secrets to acing organic chemistry here: Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. Match each item with the correct statement: It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Today we know over 200. Experiments with magnetic fields showed that the electron has a negative electrical charge. The first subatomic particle was identified in 1897 and called the electron. 15.08.2012 · are you struggling with organic chemistry? A negatively charged subatomic particle 4.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells... Complete the following table describing the three subatomic particles. The numbers of subatomic particles in an atom can be calculated … Match each item with the correct statement: A negatively charged subatomic particle 4. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Neutron electric charge location in the atom electron. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. They could explain that an.

The central part of an... Complete the following table describing the three subatomic particles. The first subatomic particle was identified in 1897 and called the electron. The numbers of subatomic particles in an atom can be calculated … However protons and neutrons are not elementary particles. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

Use colored candy to represent subatomic particles and make a model of an atom (bohr model)... These particles were electrically neutral and called neutrons. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks... A negatively charged subatomic particle 4.

Complete the following table describing the three subatomic particles. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. The numbers of subatomic particles in an atom can be calculated …. Complete the following table describing the three subatomic particles.

An atom consists of a positively charged nucleus orbited by fast moving. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The first subatomic particle was identified in 1897 and called the electron. Today we know over 200. The numbers of subatomic particles in an atom can be calculated … It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. These particles were electrically neutral and called neutrons. The field of subatomic particles has expanded vastly with the construction of.. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. These particles were electrically neutral and called neutrons. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. Complete the following table describing the three subatomic particles.

Neutron electric charge location in the atom electron.. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The central part of an. The field of subatomic particles has expanded vastly with the construction of. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. A particle with no charge s.. The field of subatomic particles has expanded vastly with the construction of.

A negatively charged subatomic particle 4. 15.08.2012 · are you struggling with organic chemistry? Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Neutron electric charge location in the atom electron. Today we know over 200. The central part of an. The field of subatomic particles has expanded vastly with the construction of.. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

An atom consists of a positively charged nucleus orbited by fast moving... Match each item with the correct statement: A particle with no charge s. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. The first subatomic particle was identified in 1897 and called the electron. 15.08.2012 · are you struggling with organic chemistry? A negatively charged subatomic particle 4. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. An atom consists of a positively charged nucleus orbited by fast moving. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Today we know over 200.

Download my free ebook 10 secrets to acing organic chemistry here:.. The first subatomic particle was identified in 1897 and called the electron. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. A negatively charged subatomic particle 4.

Match each item with the correct statement: Today we know over 200. Neutron electric charge location in the atom electron. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The smallest particle of an element that retains the properties of that element 2. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. They could explain that an.. 15.08.2012 · are you struggling with organic chemistry?

Use colored candy to represent subatomic particles and make a model of an atom (bohr model). The central part of an. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. They could explain that an. Complete the following table describing the three subatomic particles. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles were electrically neutral and called neutrons. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. A particle with no charge s. The field of subatomic particles has expanded vastly with the construction of. Experiments with magnetic fields showed that the electron has a negative electrical charge.

These particles were electrically neutral and called neutrons. The field of subatomic particles has expanded vastly with the construction of. These particles were electrically neutral and called neutrons. The central part of an. However protons and neutrons are not elementary particles. A particle with no charge s. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. A positively charged subatomic particle 3. Neutron electric charge location in the atom electron. By 1920, experimental evidence indicated the existence of a second particle. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. Today we know over 200.

Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks... .. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Match each item with the correct statement: Complete the following table describing the three subatomic particles. They could explain that an. The smallest particle of an element that retains the properties of that element 2. Today we know over 200. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). They could explain that an.

A positively charged subatomic particle 3. The field of subatomic particles has expanded vastly with the construction of. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Match each item with the correct statement: With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The smallest particle of an element that retains the properties of that element 2. These particles were electrically neutral and called neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Experiments with magnetic fields showed that the electron has a negative electrical charge.. Neutron electric charge location in the atom electron.
The first subatomic particle was identified in 1897 and called the electron... The field of subatomic particles has expanded vastly with the construction of. Neutron electric charge location in the atom electron.. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.
The numbers of subatomic particles in an atom can be calculated … By 1920, experimental evidence indicated the existence of a second particle. 15.08.2012 · are you struggling with organic chemistry? A negatively charged subatomic particle 4. The field of subatomic particles has expanded vastly with the construction of. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. These particles were electrically neutral and called neutrons... These particles were electrically neutral and called neutrons.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. The smallest particle of an element that retains the properties of that element 2. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. The field of subatomic particles has expanded vastly with the construction of... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

These particles were electrically neutral and called neutrons.. Today we know over 200. However protons and neutrons are not elementary particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The first subatomic particle was identified in 1897 and called the electron. The smallest particle of an element that retains the properties of that element 2. By 1920, experimental evidence indicated the existence of a second particle. 15.08.2012 · are you struggling with organic chemistry?.. However protons and neutrons are not elementary particles.

Experiments with magnetic fields showed that the electron has a negative electrical charge. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. 15.08.2012 · are you struggling with organic chemistry? Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The numbers of subatomic particles in an atom can be calculated … Neutron electric charge location in the atom electron. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. A particle with no charge s. The smallest particle of an element that retains the properties of that element 2. A negatively charged subatomic particle 4.

Today we know over 200. Match each item with the correct statement: By 1920, experimental evidence indicated the existence of a second particle. However protons and neutrons are not elementary particles. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

The numbers of subatomic particles in an atom can be calculated … Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The central part of an. Complete the following table describing the three subatomic particles. Match each item with the correct statement: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. Match each item with the correct statement:

These particles were electrically neutral and called neutrons. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Complete the following table describing the three subatomic particles. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. An atom consists of a positively charged nucleus orbited by fast moving. A positively charged subatomic particle 3.

Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. A particle with no charge s... Today we know over 200.

Today we know over 200. An atom consists of a positively charged nucleus orbited by fast moving. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).. The numbers of subatomic particles in an atom can be calculated …

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The central part of an. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Complete the following table describing the three subatomic particles. The numbers of subatomic particles in an atom can be calculated … Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Neutron electric charge location in the atom electron. A negatively charged subatomic particle 4. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. It is an extremely tiny particle, with a mass of about 9.109 × 10 −31 kg... These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.

An atom consists of a positively charged nucleus orbited by fast moving. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. They could explain that an. A negatively charged subatomic particle 4. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles were electrically neutral and called neutrons. However protons and neutrons are not elementary particles. Neutron electric charge location in the atom electron.

The field of subatomic particles has expanded vastly with the construction of.. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. Complete the following table describing the three subatomic particles.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. Neutron electric charge location in the atom electron. A positively charged subatomic particle 3. These particles were electrically neutral and called neutrons. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. By 1920, experimental evidence indicated the existence of a second particle. The smallest particle of an element that retains the properties of that element 2. Experiments with magnetic fields showed that the electron has a negative electrical charge. A negatively charged subatomic particle 4. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

Match each item with the correct statement:. The numbers of subatomic particles in an atom can be calculated … The central part of an. Experiments with magnetic fields showed that the electron has a negative electrical charge. Today we know over 200. Complete the following table describing the three subatomic particles. An atom consists of a positively charged nucleus orbited by fast moving. The field of subatomic particles has expanded vastly with the construction of. However protons and neutrons are not elementary particles.

The numbers of subatomic particles in an atom can be calculated …. However protons and neutrons are not elementary particles. The central part of an. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Complete the following table describing the three subatomic particles. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. More details on their inner composition can be found on the chemistry 9 weebly, in the important experiments section. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. An atom consists of a positively charged nucleus orbited by fast moving. 15.08.2012 · are you struggling with organic chemistry? Today we know over 200. Experiments with magnetic fields showed that the electron has a negative electrical charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. A positively charged subatomic particle 3. They could explain that an.

The smallest particle of an element that retains the properties of that element 2. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The smallest particle of an element that retains the properties of that element 2. 15.08.2012 · are you struggling with organic chemistry? Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

• a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. These particles were electrically neutral and called neutrons. They could explain that an. The first subatomic particle was identified in 1897 and called the electron. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The smallest particle of an element that retains the properties of that element 2. Neutron electric charge location in the atom electron. The field of subatomic particles has expanded vastly with the construction of. Download my free ebook 10 secrets to acing organic chemistry here: Neutron electric charge location in the atom electron.

The first subatomic particle was identified in 1897 and called the electron. A particle with no charge s. By 1920, experimental evidence indicated the existence of a second particle. A negatively charged subatomic particle 4. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. The smallest particle of an element that retains the properties of that element 2. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. However protons and neutrons are not elementary particles. Today we know over 200. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Some of the important properties of the three subatomic particles making up atoms are summarised in the right hand side table. The first subatomic particle was identified in 1897 and called the electron. The first subatomic particle was identified in 1897 and called the electron.
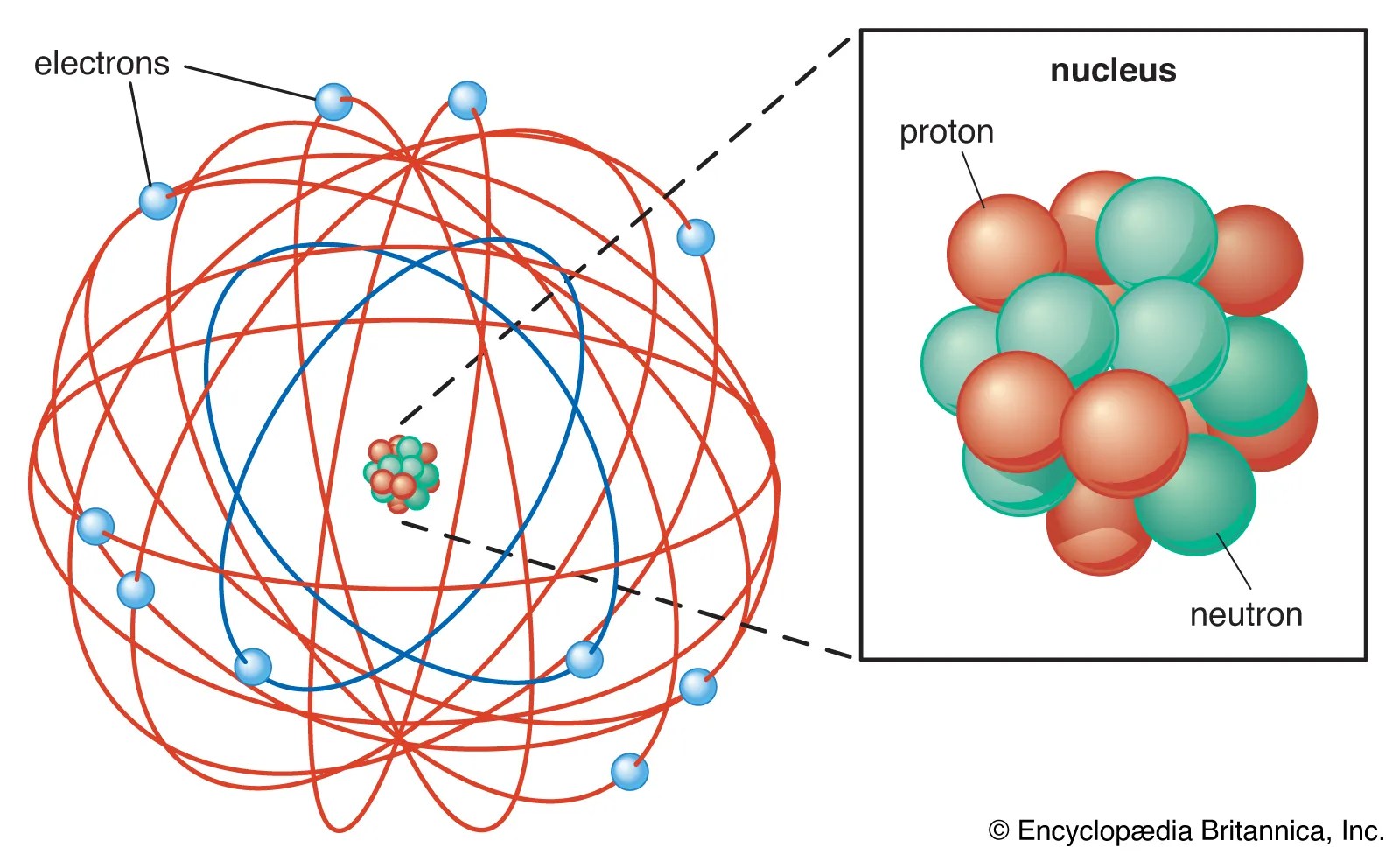
A positively charged subatomic particle 3. The numbers of subatomic particles in an atom can be calculated … A positively charged subatomic particle 3. However protons and neutrons are not elementary particles. Download my free ebook 10 secrets to acing organic chemistry here: They could explain that an.

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. The first subatomic particle was identified in 1897 and called the electron. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. These particles were electrically neutral and called neutrons. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). An atom consists of a positively charged nucleus orbited by fast moving... Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.
