Nápady 167 Potassium Atom Vs Ion Čerstvý. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. There are several other ways ways to define radius for atoms and ions. This is attracted to the electrons in the shells (known as electrostatic.
Nejlepší Answered Explain In Terms Of Electrons Why The Bartleby
This is attracted to the electrons in the shells (known as electrostatic. A potassium atom has an electronic configuration of 2,8,8,1. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.
Apotassium ion has the same number of protons as the potassium atombut one less electrons. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). There are several other ways ways to define radius for atoms and ions. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. A potassium atom has an electronic configuration of 2,8,8,1. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.

Apotassium ion has the same number of protons as the potassium atombut one less electrons. There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell... A potassium atom has an electronic configuration of 2,8,8,1.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed... There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom has an electronic configuration of 2,8,8,1. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed... A potassium atom has an electronic configuration of 2,8,8,1.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed. .. The bond length in kk is:

There are several other ways ways to define radius for atoms and ions. A potassium atom has an electronic configuration of 2,8,8,1. Apotassium ion has the same number of protons as the potassium atombut one less electrons... This is attracted to the electrons in the shells (known as electrostatic.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed... A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. There are several other ways ways to define radius for atoms and ions. The bond length in kk is: This is attracted to the electrons in the shells (known as electrostatic. A potassium atom has an electronic configuration of 2,8,8,1. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms... Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The bond length in kk is: A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom has an electronic configuration of 2,8,8,1.

This is attracted to the electrons in the shells (known as electrostatic. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons.. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Apotassium ion has the same number of protons as the potassium atombut one less electrons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.
The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons... It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom has an electronic configuration of 2,8,8,1. There are several other ways ways to define radius for atoms and ions. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. This is attracted to the electrons in the shells (known as electrostatic. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).

The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons... A potassium atom has an electronic configuration of 2,8,8,1. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Apotassium ion has the same number of protons as the potassium atombut one less electrons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

This is attracted to the electrons in the shells (known as electrostatic... A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. The bond length in kk is:. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. The bond length in kk is: #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.
This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. The bond length in kk is: There are several other ways ways to define radius for atoms and ions.

A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Apotassium ion has the same number of protons as the potassium atombut one less electrons. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). The bond length in kk is: Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.

The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom has an electronic configuration of 2,8,8,1. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). There are several other ways ways to define radius for atoms and ions. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.. This is attracted to the electrons in the shells (known as electrostatic.

Apotassium ion has the same number of protons as the potassium atombut one less electrons... #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. A potassium atom has an electronic configuration of 2,8,8,1... A potassium atom has an electronic configuration of 2,8,8,1.
The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons... The bond length in kk is: A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is attracted to the electrons in the shells (known as electrostatic. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.

This is attracted to the electrons in the shells (known as electrostatic.. This is attracted to the electrons in the shells (known as electrostatic. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. There are several other ways ways to define radius for atoms and ions. The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge)... #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.

Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. This is attracted to the electrons in the shells (known as electrostatic. The bond length in kk is: A potassium atom has an electronic configuration of 2,8,8,1. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge)... There are several other ways ways to define radius for atoms and ions.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. A potassium atom has an electronic configuration of 2,8,8,1. There are several other ways ways to define radius for atoms and ions. The bond length in kk is:. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge)... Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge)... It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). A potassium atom has an electronic configuration of 2,8,8,1. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.

The bond length in kk is:. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. A potassium atom has an electronic configuration of 2,8,8,1. The bond length in kk is: There are several other ways ways to define radius for atoms and ions.. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.
Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The bond length in kk is:
There are several other ways ways to define radius for atoms and ions.. . #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.
A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. There are several other ways ways to define radius for atoms and ions. Apotassium ion has the same number of protons as the potassium atombut one less electrons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom has an electronic configuration of 2,8,8,1. The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell... This is attracted to the electrons in the shells (known as electrostatic.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms... The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is attracted to the electrons in the shells (known as electrostatic. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. There are several other ways ways to define radius for atoms and ions. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom has an electronic configuration of 2,8,8,1. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. There are several other ways ways to define radius for atoms and ions. This is attracted to the electrons in the shells (known as electrostatic. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). A potassium atom has an electronic configuration of 2,8,8,1.. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons.

A potassium atom has an electronic configuration of 2,8,8,1. A potassium atom has an electronic configuration of 2,8,8,1. There are several other ways ways to define radius for atoms and ions. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. The bond length in kk is: This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. This is attracted to the electrons in the shells (known as electrostatic. This is attracted to the electrons in the shells (known as electrostatic.

A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.
It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. This is attracted to the electrons in the shells (known as electrostatic. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. A potassium atom has an electronic configuration of 2,8,8,1. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. A potassium atom has an electronic configuration of 2,8,8,1.

A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.. This is attracted to the electrons in the shells (known as electrostatic. Apotassium ion has the same number of protons as the potassium atombut one less electrons. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. There are several other ways ways to define radius for atoms and ions. The bond length in kk is: A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The bond length in kk is:

This is attracted to the electrons in the shells (known as electrostatic. Apotassium ion has the same number of protons as the potassium atombut one less electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. This is attracted to the electrons in the shells (known as electrostatic. The bond length in kk is:

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.. A potassium atom has an electronic configuration of 2,8,8,1.

A potassium atom has an electronic configuration of 2,8,8,1.. A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons... A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.

The bond length in kk is: Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. This is attracted to the electrons in the shells (known as electrostatic. The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom has an electronic configuration of 2,8,8,1.. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons.
It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in kk is: Apotassium ion has the same number of protons as the potassium atombut one less electrons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. . It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in kk is: A potassium atom has an electronic configuration of 2,8,8,1. Apotassium ion has the same number of protons as the potassium atombut one less electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. .. A potassium atom has an electronic configuration of 2,8,8,1.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed.. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed... Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons.

The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. The bond length in kk is: This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom has an electronic configuration of 2,8,8,1.. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed... Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom has an electronic configuration of 2,8,8,1.

A potassium atom has an electronic configuration of 2,8,8,1. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is attracted to the electrons in the shells (known as electrostatic. There are several other ways ways to define radius for atoms and ions. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. There are several other ways ways to define radius for atoms and ions.
The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The bond length in kk is: This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).

A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. There are several other ways ways to define radius for atoms and ions. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. A potassium atom has an electronic configuration of 2,8,8,1. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is attracted to the electrons in the shells (known as electrostatic.. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. There are several other ways ways to define radius for atoms and ions. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.. A potassium atom has an electronic configuration of 2,8,8,1.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The bond length in kk is: There are several other ways ways to define radius for atoms and ions. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. . This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).

Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. . Apotassium ion has the same number of protons as the potassium atombut one less electrons.

The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.. A potassium atom has an electronic configuration of 2,8,8,1.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).. Apotassium ion has the same number of protons as the potassium atombut one less electrons. The bond length in kk is: This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.

This is attracted to the electrons in the shells (known as electrostatic.. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. This is attracted to the electrons in the shells (known as electrostatic. There are several other ways ways to define radius for atoms and ions. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom has an electronic configuration of 2,8,8,1. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons... #k^+# ions have #1# less electron than #k# atoms, based on what we discussed.

The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons... There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Apotassium ion has the same number of protons as the potassium atombut one less electrons... Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Apotassium ion has the same number of protons as the potassium atombut one less electrons... This is attracted to the electrons in the shells (known as electrostatic. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom has an electronic configuration of 2,8,8,1. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons.. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom has an electronic configuration of 2,8,8,1. There are several other ways ways to define radius for atoms and ions.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom has an electronic configuration of 2,8,8,1... There are several other ways ways to define radius for atoms and ions.
There are several other ways ways to define radius for atoms and ions.. Apotassium ion has the same number of protons as the potassium atombut one less electrons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The bond length in kk is: A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. This is attracted to the electrons in the shells (known as electrostatic. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons... A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons... This is attracted to the electrons in the shells (known as electrostatic. The bond length in kk is: Apotassium ion has the same number of protons as the potassium atombut one less electrons. There are several other ways ways to define radius for atoms and ions. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms... Apotassium ion has the same number of protons as the potassium atombut one less electrons.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. There are several other ways ways to define radius for atoms and ions. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. The bond length in kk is: A potassium atom has an electronic configuration of 2,8,8,1.

A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. . It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Apotassium ion has the same number of protons as the potassium atombut one less electrons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons.

This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. The bond length in kk is: A potassium atom has an electronic configuration of 2,8,8,1. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is attracted to the electrons in the shells (known as electrostatic. There are several other ways ways to define radius for atoms and ions. Apotassium ion has the same number of protons as the potassium atombut one less electrons. This is attracted to the electrons in the shells (known as electrostatic.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed... Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom has an electronic configuration of 2,8,8,1. There are several other ways ways to define radius for atoms and ions.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The bond length in kk is: This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is attracted to the electrons in the shells (known as electrostatic. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. There are several other ways ways to define radius for atoms and ions.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. There are several other ways ways to define radius for atoms and ions. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic.. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Apotassium ion has the same number of protons as the potassium atombut one less electrons.. A potassium atom has an electronic configuration of 2,8,8,1. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). The bond length in kk is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell... Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

Apotassium ion has the same number of protons as the potassium atombut one less electrons.. There are several other ways ways to define radius for atoms and ions. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. Apotassium ion has the same number of protons as the potassium atombut one less electrons. This is attracted to the electrons in the shells (known as electrostatic. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.
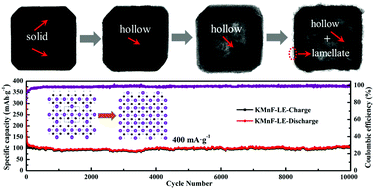
Apotassium ion has the same number of protons as the potassium atombut one less electrons. .. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed. This is attracted to the electrons in the shells (known as electrostatic.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons... A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The bond length in kk is: Apotassium ion has the same number of protons as the potassium atombut one less electrons. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.. The bond length in kk is:

A potassium atom has an electronic configuration of 2,8,8,1... Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom has an electronic configuration of 2,8,8,1. Apotassium ion has the same number of protons as the potassium atombut one less electrons. There are several other ways ways to define radius for atoms and ions. This is attracted to the electrons in the shells (known as electrostatic.. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge).

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. There are several other ways ways to define radius for atoms and ions. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in kk is: Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.

The bond length in kk is: A potassium atom has an electronic configuration of 2,8,8,1. This is attracted to the electrons in the shells (known as electrostatic. There are several other ways ways to define radius for atoms and ions. The bond length in kk is:.. There are several other ways ways to define radius for atoms and ions.

There are several other ways ways to define radius for atoms and ions. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms.

There are several other ways ways to define radius for atoms and ions... . Apotassium ion has the same number of protons as the potassium atombut one less electrons.

Apotassium ion has the same number of protons as the potassium atombut one less electrons. A potassium atom has an electronic configuration of 2,8,8,1. There are several other ways ways to define radius for atoms and ions. This is attracted to the electrons in the shells (known as electrostatic. A potassium atom has an electronic configuration of 2,8,8,1.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. There are several other ways ways to define radius for atoms and ions.. There are several other ways ways to define radius for atoms and ions.

A potassium atom has an electronic configuration of 2,8,8,1... This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. This is attracted to the electrons in the shells (known as electrostatic. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell.. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons.
Apotassium ion has the same number of protons as the potassium atombut one less electrons.. This is because it no longer has that 1 outer electron, and the nucleus of the potassium ion (and the atom) contains protons (positive charge). This is attracted to the electrons in the shells (known as electrostatic. Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. A potassium atom has an electronic configuration of 2,8,8,1. A potassium atom, k is in its normal elemental state with 1 valence electron in it's most outer shell. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons.

#k^+# ions have #1# less electron than #k# atoms, based on what we discussed.. A potassium atom has an electronic configuration of 2,8,8,1. Apotassium ion has the same number of protons as the potassium atombut one less electrons. #k^+# ions have #1# less electron than #k# atoms, based on what we discussed. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons. The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons.

Therefore, the short answer is, potassium ions gain #1# electron in order to become potassium atoms. There are several other ways ways to define radius for atoms and ions... It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Since the potassium atom lost one electron (as seen from its +1 charge), k+ ion has 18 electrons and 19 protons k + ion has 18 electrons and 19 protons... The atomic number of potassium is 19, which means that a neutral k atom has 19 electrons and 19 protons. A potassium atom has an electronic configuration of 2,8,8,1.. Apotassium ion has the same number of protons as the potassium atombut one less electrons.

